Public Alerts
The CFSRE is developing Public Alerts and related reports to increase public awareness regarding NPS involvement in adverse intoxications, mass overdoses, and fatalities. These reports are generated based on subsets of data collected at or near the time of first report or incidence and may not necessarily reflect all results for a specific emerging NPS. For more information about our Public Alerts and NPS Impacts, reach out to us via email: This email address is being protected from spambots. You need JavaScript enabled to view it..
June 15, 2022
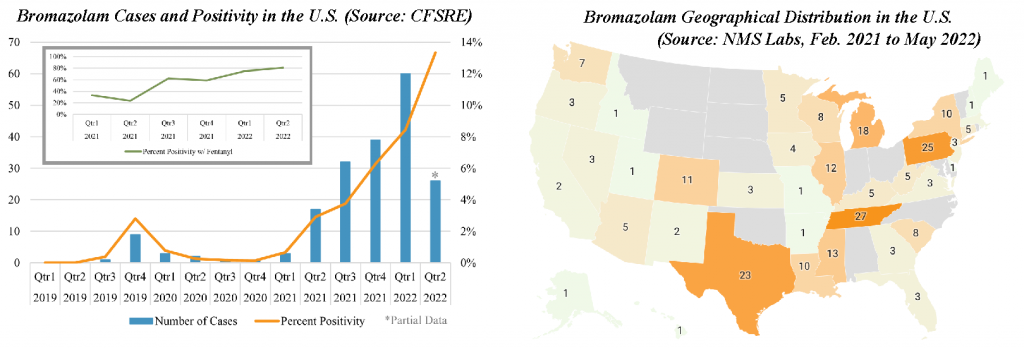
Bromazolam first emerged in the recreational drug supply in 2016 (Europe) and 2019 (United States). Bromazolam was first synthesized during medicinal drug development in the 1970s but never approved for therapeutic use in the United States. Bromazolam is the brominated counterpart to the chlorinated drug alprazolam. Bromazolam has been linked to adverse events resulting in hospitalization and death. Bromazolam is commonly reported in combination with other drugs, including the opioid fentanyl. To date, bromazolam has been identified in more than 250 toxicology cases submitted to NMS Labs, including both antemortem and postmortem investigations. Bromazolam has been identified in more than 190 toxicology samples tested at the Center for Forensic Science Research and Education (CFSRE), displaying an increase in positivity from 1% in Q1 2021 to 13% in Q2 2022. More significantly, co-detections with fentanyl have increased in recent months to more than 75% for bromazolam positive samples. Bromazolam has also been confirmed in counterfeit benzodiazepine preparations at the CFSRE.
Download the Alert
Download the Alert
April 20, 2022
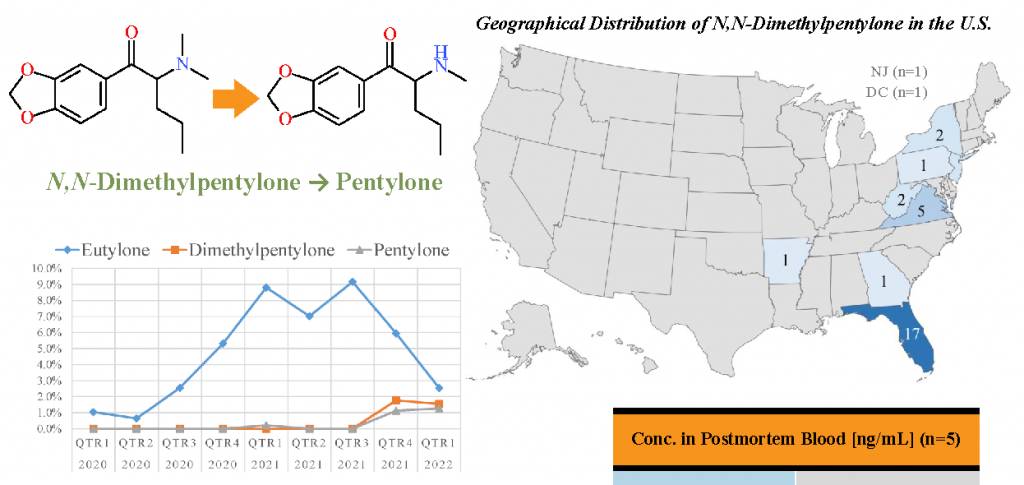
In 2020 and 2021, the substituted cathinone eutylone was the most commonly encountered synthetic stimulant to appear in forensic casework, despite the drug being considered federally scheduled as an isomer of pentylone since March 2017 according to the U.S. Drug Enforcement Administration (DEA). In September 2021, eutylone was recommended for international control. It is this notice that likely created a shift in the NPS drug market, which would later be noted by declining eutylone positivity and increasing N,N-dimethylpentylone positivity. N,N-Dimethylpentylone was first identified in toxicology samples in the U.S. in Q3 2021, marking the initial insurgence of this drug into the supply and the beginning of its proliferation. To date, N,N-dimethylpentylone has been identified in 32 toxicology cases, including antemortem and postmortem investigations, in addition to drug material cases. N,N-Dimethylpentylone is not explicitly scheduled in the U.S.; however, it could be considered an isomer of N-ethyl pentylone (Schedule I). Of note, pentylone is a metabolite of N,N-dimethylpentylone.
Download the Alert
Download the Alert
December 14, 2021
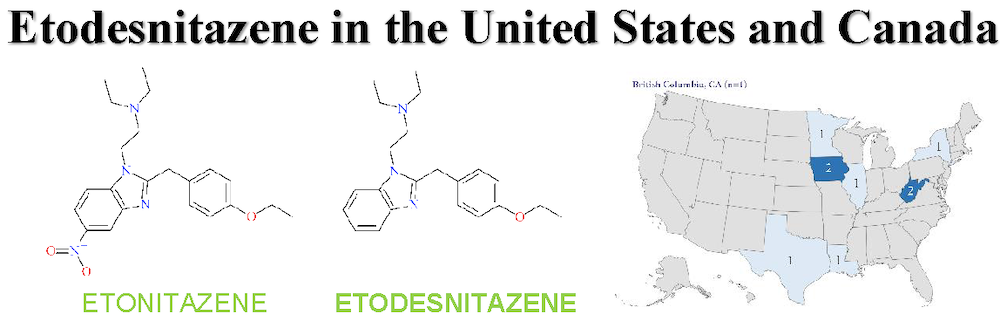
Etodesnitazene is a new synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid that is nationally and internationally controlled. Etodesnitazene is dissimilar in structure to synthetic opioids typically encountered in forensic casework (e.g., fentanyl, heroin); however, several analogues of this series (known as “nitazenes”) have recently emerged in several countries around the world. In vitro pharmacological data show that etodesnitazene is an active opioid agonist which is approximately four times less potent than fentanyl (a common phenomenon for analogues missing the 5-nitro group) but approximately six times more potent than morphine. Etodesnitazene was first reported by NPS Discovery in February 2021 following initial detection in a toxicology case. To date, ten blood and/or urine specimens associated with postmortem death investigations or clinical intoxications in the United States and Canada were confirmed to contain etodesnitazene. Identifications of etodesnitazene have also been reported from organizations in Europe. The toxicity of etodesnitazene has not been examined or reported but recent association with death among people who use drugs leads professionals to believe this synthetic opioid retains the potential to cause harm and is of public health concern.
DOWNLOAD THE ALERT
DOWNLOAD THE ALERT
December 10, 2021
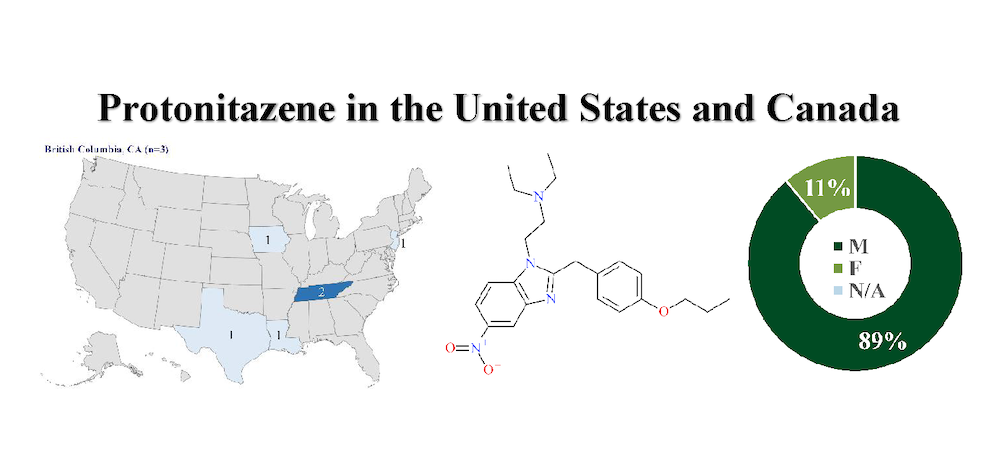
Protonitazene is a new, potent synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid that is nationally and internationally controlled. Protonitazene is dissimilar in structure to synthetic opioids typically encountered in forensic casework (e.g., fentanyl, heroin); however, protonitazene is a structural isomer of isotonitazene, requiring increased analytical specificity during toxicological analysis. In vitro pharmacological data suggest that this new opioid exhibits potency similar to other recently emergent “nitazene” opioids, and is approximately three times more potent than fentanyl. Protonitazene was first reported by NPS Discovery in May 2021 following initial detection in a toxicology case. To date, nine blood specimens associated with postmortem death investigations in the U.S. were confirmed to contain protonitazene; however, at least six additional cases have been discovered through toxicological surveillance by NPS Discovery as of December 2021. Identifications of protonitazene have also been reported from organizations in Europe. The toxicity of protonitazene has not been examined or reported but recent association with death among people who use drugs leads professionals to believe this synthetic opioid retains the potential to cause widespread harm and is of public health concern.
DOWNLOAD THE ALERT
DOWNLOAD THE ALERT
December 3, 2021
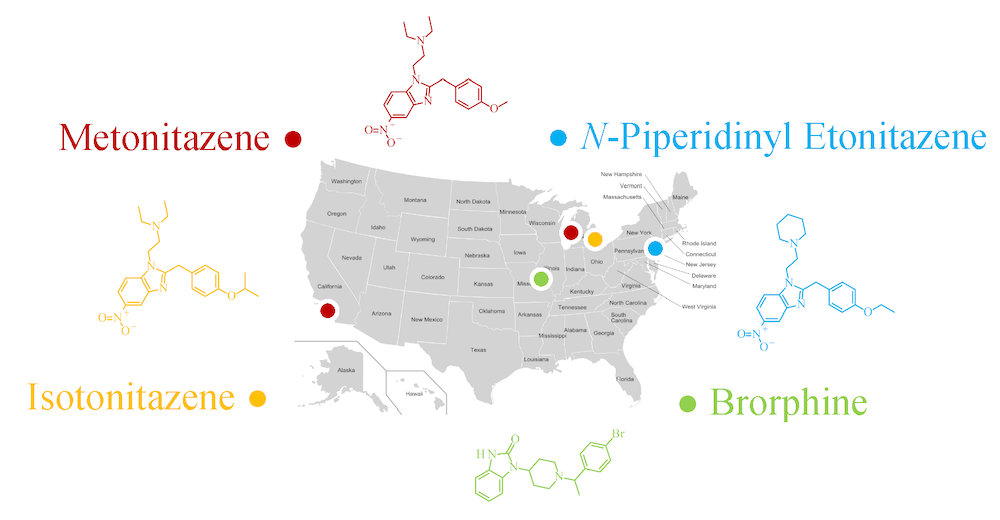
The objective of this announcement is to notify public health and safety, clinicians, law enforcement, first responders, medical examiners and coroners, forensic and clinical laboratory personnel, and all other related communities about new information surrounding new generation synthetic opioids in clinical settings after suspected opioid overdoses and presentation to emergency departments, including: metonitazene, N-piperidinyl etonitazene, isotonitazene, and brorphine.
Drug use can lead to adverse events and overdose scenarios where individuals present to emergency departments for clinical evaluation and/or treatment. The culprit can be traditional drugs (e.g., heroin, fentanyl, cocaine, methamphetamine) or novel psychoactive substances (NPS); however, proper drug testing methodologies must be employed for accurate identification and characterization. A partnership between the American College of Medical Toxicology (ACMT) and the Center for Forensic Science Research and Education (CFSRE) was established to comprehensively assess the role and prevalence of synthetic opioids and other drugs among suspected overdose events in the United States. Patients with a suspected opioid overdose presented to an emergency department at a participating site within ACMT’s Toxicology Investigators Consortium (ToxIC). Residual, discarded biological samples were obtained for testing against an expansive library of drugs and other substances.
DOWNLOAD THE ALERT
Drug use can lead to adverse events and overdose scenarios where individuals present to emergency departments for clinical evaluation and/or treatment. The culprit can be traditional drugs (e.g., heroin, fentanyl, cocaine, methamphetamine) or novel psychoactive substances (NPS); however, proper drug testing methodologies must be employed for accurate identification and characterization. A partnership between the American College of Medical Toxicology (ACMT) and the Center for Forensic Science Research and Education (CFSRE) was established to comprehensively assess the role and prevalence of synthetic opioids and other drugs among suspected overdose events in the United States. Patients with a suspected opioid overdose presented to an emergency department at a participating site within ACMT’s Toxicology Investigators Consortium (ToxIC). Residual, discarded biological samples were obtained for testing against an expansive library of drugs and other substances.
DOWNLOAD THE ALERT
August 31, 2021
Cayman Chemical and NPS Discovery at the Center for Forensic Science Research and Education (CFSRE) have developed new, more appropriate names to address the newly emergent “MDA-19” and its related analogues following the systematic convention typically used for synthetic cannabinoid nomenclature.
Synthetic cannabinoids represent a vastly diverse sub-class of novel psychoactive substances (NPS). The turnover of this sub-class is largely linked to drug scheduling actions and, like other sub-classes of NPS, new drugs were historically produced via slight tweaks to the molecular structure. In May 2021, China announced new legislation to control synthetic cannabinoids as a class using commonly encountered structural backbones. This has resulted in the emergence of new generations of synthetic cannabinoids with core components that were previously unencountered and/or not well characterized. An example is “MDA-19” and its related analogues. “MDA-19” is a CB2 agonist and was studied, like many synthetic cannabinoids, under legitimate research for pharmaceutical purposes. Similar to JWH-018 and other early synthetic cannabinoids, naming conventions utilizing the initials of a researcher or organization are not ideal and may be misleading (e.g., the abbreviation “MDA” is also used for the stimulant drug methylenedioxyamphetamine). A well-accepted systematic naming convention exists for synthetic cannabinoids and should be applied, where appropriate, to avoid any confusion or mischaracterization.
DOWNLOAD THE ALERT
Synthetic cannabinoids represent a vastly diverse sub-class of novel psychoactive substances (NPS). The turnover of this sub-class is largely linked to drug scheduling actions and, like other sub-classes of NPS, new drugs were historically produced via slight tweaks to the molecular structure. In May 2021, China announced new legislation to control synthetic cannabinoids as a class using commonly encountered structural backbones. This has resulted in the emergence of new generations of synthetic cannabinoids with core components that were previously unencountered and/or not well characterized. An example is “MDA-19” and its related analogues. “MDA-19” is a CB2 agonist and was studied, like many synthetic cannabinoids, under legitimate research for pharmaceutical purposes. Similar to JWH-018 and other early synthetic cannabinoids, naming conventions utilizing the initials of a researcher or organization are not ideal and may be misleading (e.g., the abbreviation “MDA” is also used for the stimulant drug methylenedioxyamphetamine). A well-accepted systematic naming convention exists for synthetic cannabinoids and should be applied, where appropriate, to avoid any confusion or mischaracterization.
DOWNLOAD THE ALERT
June 17, 2021
N-Pyrrolidino etonitazene (etonitazepyne) is a new high potency synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid that is nationally and internationally controlled. N-Pyrrolidino etonitazene is dissimilar in structure to other synthetic opioids typically encountered in forensic casework (e.g., fentanyl). Unlike the 2-benzylbenzimidazole analogues that were first synthesized and reported in the literature in the 1950s (e.g., metonitazene, isotonitazene), N-pyrrolidino etonitazene does not appear in prior literature or patents. Recent in vitro pharmacological data suggest that this new opioid exhibits potency similar to etonitazene (~20x more potent than fentanyl). N-Pyrrolidino etonitazene was first reported by NPS Discovery in May 2021 following initial detection in a toxicology case. To date, eight blood specimens associated with postmortem death investigations in the U.S. have contained N-pyrrolidino etonitazene; additional confirmations are pending. The toxicity of N-pyrrolidino etonitazene has not been examined or reported but recent association with death among people who use drugs leads professionals to believe this synthetic opioid retains the potential to cause widespread harm and is of public health concern. Identifications of N-pyrrolidino etonitazene have also been reported recently from agencies in Europe.
DOWNLOAD THE ALERT
DOWNLOAD THE ALERT
May 28, 2021
Tramadol is a synthetic opioid analgesic approved in 1995 by the United States Food and Drug Administration (FDA) for moderate to moderately severe pain. Its mechanism of action involves opioid and non-opioid pharmacological activities. Tramadol is a μ-opioid receptor agonist, producing opioid-like effects of analgesia, and respiratory and CNS depression. It also acts as a serotonin and norepinephrine reuptake inhibitor, which can cause excitatory neurological effects. Tramadol is metabolized by CYP2D6 to O-desmethyltramadol, an active metabolite that is more potent and has a longer half-life than the parent drug. Tramadol is approximately equipotent to its structural analog codeine. In 2014, the FDA classified tramadol as a schedule IV controlled substance due to its potential for abuse. Tramadol is available as immediate-release and extended-release tablets. Trade names include ConZip®, Ultram®, Ultracet®, Ryzolt®, and Rybix ODT®.
DOWNLOAD THE ALERT
DOWNLOAD THE ALERT
May 25, 2021
Dipyrone is a non-opioid analgesic with antipyretic activity, which was developed by the German company Hoechst AG in 1920 with mass production starting in 1922. It is a pro-drug, which is rapidly metabolized after oral administration to active pyrazolone compounds. Dipyrone is also know under different generic names such as metamizole, noramidopyrine, and others. Dipyrone was sold as an over-the-counter (OTC) analgesic until the 1970s, at which time it was banned in several countries, including the United States, several European nations, Japan, and Australia following reports of users developing agranulocytosis, occasionally resulting in death. The safety of the drug is still controversial, resulting in varying levels of restriction and regulation worldwide. Dipyrone is still available, however, by prescription and OTC in many countries in Europe, South America, and Asia.
DOWNLOAD THE ALERT
DOWNLOAD THE ALERT
May 18, 2021
The objective of this report is to provide guidance in developing an appropriate analytical scope of testing for novel psychoactive substances (NPS) in the United States based on current trends and intelligence. This report is based on information available in Q1 2021 and is subject to change along with the drug market.
NPS Discovery and the SOFT Designer Drugs Committee have established the below recommendations for NPS scope based on information from extensive collaborations, partnerships, and initiatives which yield national perspectives. Suggested cut-off concentrations or reporting limits (in ng/mL) are listed for each NPS. These values were categorized (i.e., <1, 1-10, and >10 ng/mL) and determined based on currently available quantitative data and/or comparison to structurally similar NPS within the given sub-class.
DOWNLOAD THE ALERT
NPS Discovery and the SOFT Designer Drugs Committee have established the below recommendations for NPS scope based on information from extensive collaborations, partnerships, and initiatives which yield national perspectives. Suggested cut-off concentrations or reporting limits (in ng/mL) are listed for each NPS. These values were categorized (i.e., <1, 1-10, and >10 ng/mL) and determined based on currently available quantitative data and/or comparison to structurally similar NPS within the given sub-class.
DOWNLOAD THE ALERT